Explain How 4p Orbitals Are Different From 3p Orbitals.
Thus a 2p orbital has 1 node and a 3p orbital has 2 nodes. Or does the whole atom have 4.
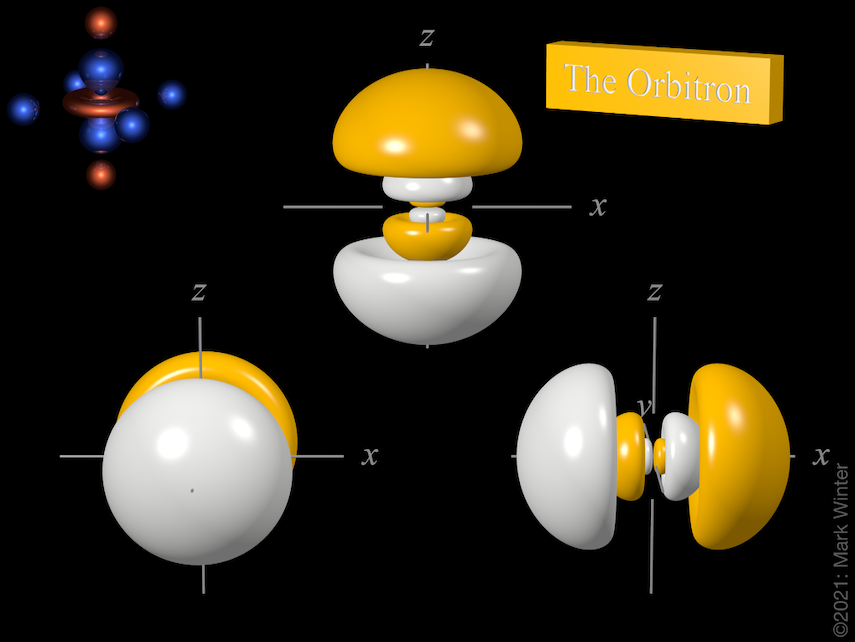
The Orbitron 4p Atomic Orbitals
The orbital would be the same shape as the orbital but would be smaller in size.
. Thus all orbitals with designation 3 have 3 zones where the probability of finding an electron is zero. Explain how 4p orbitals are different from 3p orbitals. 4p orbitals are larger in size than 3p orbitals and contain no nodes.
1s 2s 2p 3s 3p 3d 4p 4d 4f. 8 What is the difference in the orbital angular momentum of 2p and 3p electron explain. Up to 24 cash back The three p orbitals differ only in their orientation and are orthogonal to one another.
How would the 2s and 3p orbitals differ from the 1s and 2p orbitals. Now come towards your question as you ask about difference between 2p and 3p orbital the answer is that 3p orbital has same structureshape that of 2p orbital has but larger in size and energybecause it lies in 3rd orbit of an atom similarly 4p orbitals will have same shape but higher energy and larger in size but shape will be similarsimilarly 5p orbital and. Hence we can say that there are five d-orbitals.
Similar to s orbitals size and energy of p orbitals increases with an increase in the principal quantum number 4p 3p 2p. Also orbitals does each shell have 4 orbitals. 4p orbitals are larger in size than 5p orbitals and contain additional nodes.
By the Aufbau principle 3p will be filled first before 4p. The 3p orbitals have the same general shape and are larger than 2p orbitals but they differ in the number of nodes. Orbitals can be ranked in the increasing order of orbital energy as follows.
An orbital can contain a maximum of two electrons. The Shape of d Orbitals. Ο Ο Ο O 5p orbitals are larger in size than 4p orbitals and contain no nodes.
In the case of hydrogen the orbital which is called 1s is the one which is occupied by the hydrogen electron. That single electron does not introduce orbital anguar momentum so no matter what value of l the orbital energies for the same n are all the same. The added electrons in multi-electron atoms are intrinsically correlated in such a way that each electron is influenced by the motions of the others.
Possible answers The and orbitals would have more nodes than and orbitals. The third energy level has 3s x1. Item 60 Part A Explain how 5p orbitals are different from 4p orbitals.
4p orbitals are larger in size than 3p orbitals and contain less nodes. 5p orbitals are larger in size than 4p orbitals and contain less nodes. The same shape as.
Tap again to see term. 4p orbitals are larger in size than 3p orbitals and contain additional nodes. O 3p orbitals are larger in size than 4p orbitals and contain additional nodes.
Check all that apply. The aufbau principle explains how electrons fill low energy orbitals closer to the nucleus before they fill higher energy ones. Thus the s sublevel which has only one orbital can have only two electrons.
A different shape than. Basically how do you determine which shell is valenceCuz like oxygen for instance 6 electron outside 2 inside but on charts it says valence is 2. Also the 2s and 3p orbitals would.
The 3p 4p 5p and higher p orbitals are all similar in shape to the 2p orbitals but they contain additional nodes as same as higher s orbitals and are progressively. Can it be less or more. Similar patterns are followed in other sublevels as well.
Chemistry questions and answers. The 2s and 3p orbitals would have more nodes than 1s and 2p orbitalsWhat are two differences between a 2p and a 3p orbitalThe. Nodes can be either angular or radial.
Do all elements have 4. The 2s orbital would be the same shape as the 1s orbital but would be smaller in size and the 3p orbitals would have a different shape than the 2p orbitals but would be larger in size. This is because of the.
Or what Also does anyone know a good video or visual way to show. 3p x 3 and 3d x 5 however the 3d orbitals have an energy which actually places them in the fourth energy level between 4s and 4p. Here 1represents the first.
Because of how there IS only one electron in H atom. 9 What is the shape of a 3p orbital. Tap card to see definition.
Region of space where a high probability exists for finding an electron. The same amount of. The magnetic orbital quantum number for d orbitals is given as -2-10 12.
However the energy of an electron in multi-electron atoms depends on both on its principal quantum number n and its azimuthal quantum number l. Click card to see definition. 3d has 2 planar nodes giving it the familiar 4-lobed shape.
The 1s orbital is a sphere and the 2p orbital is made up of three dumbbells oriented in the x y and z direction. It is convenient to define an x y and z axis system and then label each p orbital as px py and pz. You have probably noticed that the total number of nodes in an orbital is equal to n 1 where n is the principal quantum number.
Tap card to see definition. Also the 2s and 3p orbitals would have more nodes. Where there is a choice between orbitals of equal energy they fill the orbitals singly as far as possible Hunds rulesThe diagram not to scale summarizes the energies of the orbitals up to the 4p level.
The Order of Filling Orbitals. The p sublevels are named 2p 3p and 4p since the p sublevel appears only starting the 2nd level. Now come towards your question as you ask about difference between 2p and 3p orbital the answer is that 3p orbital has same structureshape that of 2p orbital has but larger in size and energybecause it lies in 3rd orbit of an atom similarly 4p orbitals will have same shape but higher energy and larger in size but shape will be similarsimilarly 5p orbital and.
I dont fully understand them. Click again to see term. 10 How would the 2s 2 S and 3p.
3s has 2 spherical nodes the 3rd zone is infinite distance from the atom 3p has one planar and 1 spherical node. The 2s orbital would be the same shape as the 1s orbital but would be larger in size and the 3p orbital would have the same shape as the 2p orbitals bout would be larger in size. These orbitals are designated as d xy d yz d xz d x 2 y 2.
Click card to see definition.

Solved Explain How 4p Orbitals Are Dilierent From 3p Orbilals 4p Orbitals Are Larger In Size Han 3p Orbitals And Contain Less Nodes 4p Orbilals Are Larger In Size Than 3p Orbitals And

The Gvb 3p Lobe Orbitals 3p Z 3p Z And The 3p X P Download Scientific Diagram

Solved Explain How 4p Orbitals Are Different From 3p Chegg Com
Why Are The Shapes Of 2p And 3p Orbitals Different In One Picture I Saw Two More Small Lobes Between Two Big Lobes Here Is The Picture Quora
0 Response to "Explain How 4p Orbitals Are Different From 3p Orbitals."
Post a Comment